Photosynthetica 2023, 61(4):451-460 | DOI: 10.32615/ps.2023.037
Growth light substantially affects both primary and secondary metabolic processes in Catharanthus roseus plants
- 1 Department of Plant Physiology and Metabolomics, Agricultural Institute, Centre for Agricultural Research, H-2462 Brunszvik u. 2., Martonvásár, Hungary
- 2 Department of Plant Biotechnology, National Research Centre, 33 El Behouth St. (former El-Tahrir St.), Dokki, P.O. 12622 Giza, Egypt
- 3 Department of Horticultural Science, Faculty of Agriculture, University of Maragheh, Maragheh, Iran
- 4 Center for Research and Technology Transfer (CRETECH), Vietnam Academy of Sciences and Technology (VAST), 10072 Hanoi, Vietnam
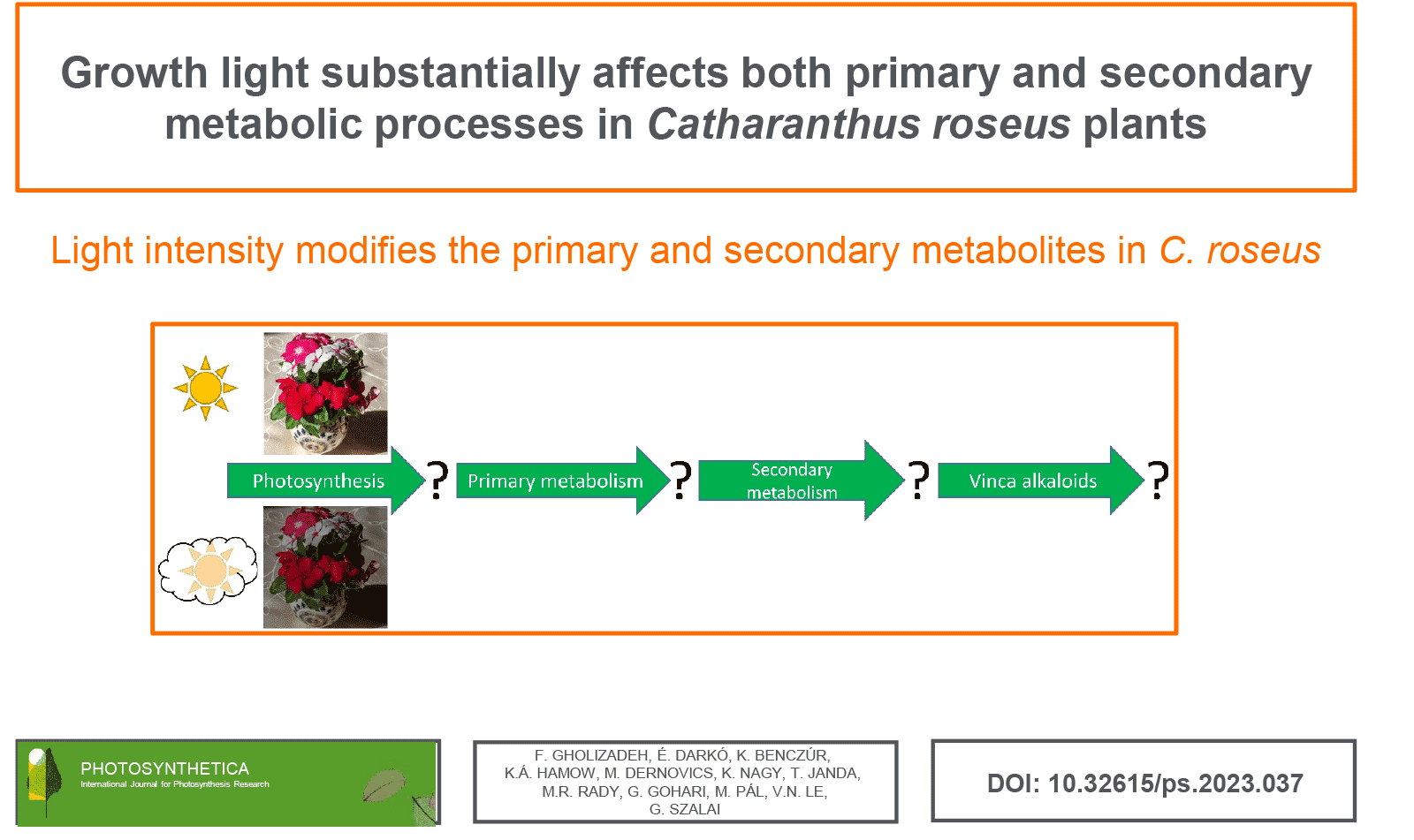
Common periwinkle (Catharanthus roseus L.) is an important medicinal plant used by the pharmaceutical industry. The present work aimed to determine the effect of low light intensity on the primary and secondary metabolic processes, using various photosynthesis and targeted and untargeted analytical techniques. Growth light had only limited effects on the photosynthetic electron transport processes, although membrane stability seemed slightly higher in plants growing under higher light conditions. The reduced growth light caused a reduction in certain primary metabolites, including amino acids and sugars, and it also reduced the contents of most of the phenolic compounds investigated in the present experiments. Interestingly, the differences in the growth light caused a much less pronounced difference in the alkaloid contents than that found in the flavonoid contents. However, besides the growth light, genotypic differences, most evident in flower colour, also affected some metabolic processes, including primary and secondary processes.
Additional key words: acclimation processes; flower colour; growth light; metabolomics; periwinkle; vinca alkaloids.
Received: July 24, 2023; Revised: September 6, 2023; Accepted: September 26, 2023; Prepublished online: October 10, 2023; Published: December 19, 2023 Show citation
| ACS | AIP | APA | ASA | Harvard | Chicago | Chicago Notes | IEEE | ISO690 | MLA | NLM | Turabian | Vancouver |
Supplementary files
| Download file | Gholizadeh_3047_supplement.pdf File size: 2.41 MB |
References
- Akhgari A., Laakso I., Seppänen-Laakso T. et al.: Analysis of indole alkaloids from Rhazya stricta hairy roots by ultra-performance liquid chromatography-mass spectrometry. - Molecules 20: 22621-22634, 2015.
 Go to original source...
Go to original source... - Asano M., Harada K., Yoshikawa T. et al.: Synthesis of anti-tumor dimeric indole alkaloids in Catharanthus roseus was promoted by irradiation with near-ultraviolet light at low temperature. - Biosci. Biotech. Bioch. 74: 386-389, 2010.
 Go to original source...
Go to original source... - Badmus U.O., Crestani G., Cunningham N. et al.: UV radiation induces specific changes in the carotenoid profile of Arabidopsis thaliana. - Biomolecules 12: 1879, 2022.
 Go to original source...
Go to original source... - Bassi R., Dall'Osto L.: Dissipation of light energy absorbed in excess: the molecular mechanisms. - Annu. Rev. Plant Biol. 72: 47-76, 2021.
 Go to original source...
Go to original source... - Bhutkar M.A., Bhise S.B.: Comparative studies on antioxidant properties of Catharanthus rosea and Catharanthus alba. - Int. J. Pharmtech. Res. 3: 1551-1556, 2011.
- Boccia M., Grzech D., Lopes A.A. et al.: Directed biosynthesis of new to nature alkaloids in a heterologous Nicotiana benthamiana expression host. - Front. Plant Sci. 13: 919443, 2022.
 Go to original source...
Go to original source... - Croce R.: Beyond 'seeing is believing': the antenna size of the photosystems in vivo. - New Phytol. 228: 1214-1218, 2020.
 Go to original source...
Go to original source... - Darkó É., Khalil R., Elsayed N. et al.: Factors playing role in heat acclimation processes in barley and oat plants. - Photosynthetica 57: 1035-1043, 2019.
 Go to original source...
Go to original source... - De Luca V., Balsevich J., Tyler R.T. et al.: Biosynthesis of indole alkaloids: developmental regulation of the biosynthetic pathway from tabersonine to vindoline in Catharanthus roseus. - J. Plant Physiol. 125: 147-156, 1986.
 Go to original source...
Go to original source... - Eng J.G.M., Shahsavarani M., Smith D.P. et al.: A Catharanthus roseus Fe(II)/α-ketoglutarate-dependent dioxygenase catalyzes a redox-neutral reaction responsible for vindolinine biosynthesis. - Nat. Commun. 13: 3335, 2022.
 Go to original source...
Go to original source... - Galili G., Avin-Wittenberg T., Angelovici R., Fernie A.R.: The role of photosynthesis and amino acid metabolism in the energy status during seed development. - Front. Plant Sci. 5: 447, 2014.
 Go to original source...
Go to original source... - Ghasemzadeh A., Jaafar H.Z.E., Rahmat A. et al.: Effect of different light intensities on total phenolics and flavonoids synthesis and anti-oxidant activities in young ginger varieties (Zingiber officinale Roscoe). - Int. J. Mol. Sci. 11: 3885-3897, 2010.
 Go to original source...
Go to original source... - Gondor O.K., Tajti J., Hamow K.Á. et al.: Polyamine metabolism under different light regimes in wheat. - Int. J. Mol. Sci. 22: 11717, 2021.
 Go to original source...
Go to original source... - Havaux M., Tardy F., Ravenel J. et al.: Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: influence of the xanthophyll content. - Plant Cell Environ. 19: 1359-1368, 1996.
 Go to original source...
Go to original source... - Janda T., Prerostová S., Vanková R., Darkó É.: Crosstalk between light- and temperature-mediated processes under cold and heat stress conditions in plants. - Int. J. Mol. Sci. 22: 8602, 2021a.
 Go to original source...
Go to original source... - Janda T., Tajti J., Hamow K.Á. et al.: Acclimation of photosynthetic processes and metabolic responses to elevated temperatures in cereals. - Physiol. Plantarum 171: 217-231, 2021b.
 Go to original source...
Go to original source... - Klughammer C., Schreiber U.: Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. - PAM Application Notes 1: 27-35, 2008.
- Krieger-Liszkay A.: Singlet oxygen production in photosynthesis. - J. Exp. Bot. 56: 337-346, 2005.
 Go to original source...
Go to original source... - Levin G., Schuster G.: LHC-like proteins: the guardians of photosynthesis. - Int. J. Mol. Sci. 24: 2503, 2023.
 Go to original source...
Go to original source... - Levin G., Yasmin M., Liveanu V. et al.: A desert Chlorella sp. that thrives at extreme high-light intensities using a unique photoinhibition protection mechanism. - Plant J. 115: 510-528, 2023.
 Go to original source...
Go to original source... - Li L., Aro E.-M., Millar A.H.: Mechanisms of photodamage and protein turnover in photoinhibition. - Trends Plant Sci. 23: 667-676, 2018.
 Go to original source...
Go to original source... - Liu Y., Zhao D.-M., Zu Y.-G. et al.: Effects of low light on terpenoid indole alkaloid accumulation and related biosynthetic pathway gene expression in leaves of Catharanthus roseus seedlings. - Bot. Stud. 52: 191-196, 2011.
- Livak K.J., Schmittgen T.D.: Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. -Methods 25: 402-408, 2001.
 Go to original source...
Go to original source... - Loris E.A., Panjikar S., Ruppert M. et al.: Structure-based engineering of strictosidine synthase: auxiliary for alkaloid libraries. - Chem. Biol. 14: 979-985, 2007.
 Go to original source...
Go to original source... - Nosalewicz A., Okoń K., Skorupka M.: Non-photochemical quenching under drought and fluctuating light. - Int. J. Mol. Sci. 23: 5182, 2022.
 Go to original source...
Go to original source... - Okazawa T., Nishijima T.: Effect of low light intensity on longevity of flowering on bedding plants targeted for indoor use. - Jpn. Agr. Res. Q. 51: 279-286, 2017.
 Go to original source...
Go to original source... - Pál M., Hamow K.Á., Rahman A. et al.: Light spectral composition modifies polyamine metabolism in young wheat plants. - Int. J. Mol. Sci. 23: 8394, 2022.
 Go to original source...
Go to original source... - Pál M., Ivanovska B., Oláh T. et al.: Role of polyamines in plant growth regulation of Rht wheat mutants. - Plant Physiol. Biochem. 137: 189-202, 2019.
 Go to original source...
Go to original source... - Pan J., Guo B.: Effects of light intensity on the growth, photosynthetic characteristics, and flavonoid content of Epimedium pseudowushanense B.L.Guo. - Molecules 21: 1475, 2016.
 Go to original source...
Go to original source... - Pan Q., Wang C., Xiong Z. et al.: CrERF5, an AP2/ERF transcription factor, positively regulates the biosynthesis of bisindole alkaloids and their precursors in Catharanthus roseus. - Front. Plant Sci. 10: 931, 2019.
 Go to original source...
Go to original source... - Rady M.R., Gierczik K., Ibrahem M.M. et al.: Anticancer compounds production in Catharanthus roseus by methyl jasmonate and UV-B elicitation. - S. Afr. J. Bot. 142: 34-41, 2021.
 Go to original source...
Go to original source... - Rahman A., Tajti J., Majláth I. et al.: Influence of a phyA mutation on polyamine metabolism in Arabidopsis depends on light spectral conditions. - Plants-Basel 12: 1689, 2023.
 Go to original source...
Go to original source... - Ruban A., Wilson S.: The mechanism of non-photochemical quenching in plants: localization and driving forces. - Plant Cell Physiol. 62: 1063-1072, 2021.
 Go to original source...
Go to original source... - Sander G.W.: Quantitative analysis of metabolic pathways in Catharanthus roseus hairy roots metabolically engineered for terpenoid indole alkaloid overproduction. PhD Thesis. Pp. 285. Iowa State University, Ames 2009.
- Sharma A., Verma P., Mathur A., Mathur A.K.: Overexpression of tryptophan decarboxylase and strictosidine synthase enhanced terpenoid indole alkaloid pathway activity and antineoplastic vinblastine biosynthesis in Catharanthus roseus. - Protoplasma 255: 1281-1294, 2018.
 Go to original source...
Go to original source... - Sharma N., Nagar S., Thakur M. et al.: Photosystems under high light stress: throwing light on mechanism and adaptation. - Photosynthetica 61: 250-263, 2023.
 Go to original source...
Go to original source... - Stöckigt J., Barleben L., Panjikar S., Loris E.A.: 3D-structure and function of strictosidine synthase - the key enzyme of monoterpenoid indole alkaloid biosynthesis. - Plant Physiol. Biochem. 46: 340-355, 2008.
 Go to original source...
Go to original source... - Sutulienė R., Lauľikė K., Pukas T., Samuolienė G.: Effect of light intensity on the growth and antioxidant activity of sweet basil and lettuce. - Plants-Basel 11: 1709, 2022.
 Go to original source...
Go to original source... - Tang W., Guo H., Baskin C.C. et al.: Effect of light intensity on morphology, photosynthesis and carbon metabolism of alfalfa (Medicago sativa) seedlings. - Plants-Basel 11: 1688, 2022.
 Go to original source...
Go to original source... - Thoma F., Somborn-Schulz A., Schlehuber D. et al.: Effects of light on secondary metabolites in selected leafy greens: a review. - Front. Plant Sci. 11: 497, 2020.
 Go to original source...
Go to original source... - Utasi L., Kovács V., Gulyás Z. et al.: Threshold or not: Spectral composition and light-intensity dependence of growth and metabolism in tomato seedlings. - Sci. Hortic.-Amsterdam 313: 111946, 2023.
 Go to original source...
Go to original source... - van der Heijden R., Jacobs D.I., Snoeijer W. et al.: The Catharanthus alkaloids: pharmacognosy and biotechnology. - Curr. Med. Chem. 11: 607-628, 2004.
 Go to original source...
Go to original source... - Vázquez-Flota F.A., De Luca V.: Jasmonate modulates development- and light-regulated alkaloid biosynthesis in Catharanthus roseus. - Phytochemistry 49: 395-402, 1998.
 Go to original source...
Go to original source... - Vázquez-Flota F.A., St-Pierre B., De Luca V.: Light activation of vindoline biosynthesis does not require cytomorphogenesis in Catharanthus roseus seedlings. - Phytochemistry 55: 531-536, 2000.
 Go to original source...
Go to original source... - Vecchi V., Barera S., Bassi R., Dall'Osto L.: Potential and challenges of improving photosynthesis in algae. - Plants-Basel 9: 67, 2020.
 Go to original source...
Go to original source... - Vredenberg W.J.: On the quantitative relation between dark kinetics of NPQ-induced changes in variable fluorescence and the activation state of the CF0.CF1.ATPase in leaves. - Photosynthetica 56: 139-149, 2018.
 Go to original source...
Go to original source... - Vrhovsek U., Masuero D., Gasperotti M. et al.: A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. - J. Agr. Food Chem. 60: 8831-8840, 2012.
 Go to original source...
Go to original source... - Wong S.L., Huang M.Y., Chen C.W., Weng J.H.: Light induction of nonphotochemical quenching, CO2 fixation, and photoinhibition in woody and fern species adapted to different light regimes. - Photosynthetica 52: 272-280, 2014.
 Go to original source...
Go to original source... - Xu M.Y., Wu K.X., Liu Y. et al.: Effects of light intensity on the growth, photosynthetic characteristics, and secondary metabolites of Eleutherococcus senticosus Harms. - Photosynthetica 58: 881-889, 2020.
 Go to original source...
Go to original source...




