Photosynthetica 2024, 62(2):147-157 | DOI: 10.32615/ps.2024.014
Using chlorophyll fluorescence parameters and antioxidant enzyme activity to assess drought tolerance of spring wheat
- 1 Siberian Institute of Plant Physiology and Biochemistry SB RAS, 664033 Irkutsk, Russia
- 2 Faculty of Biology and Soil, Irkutsk State University, 664003 Irkutsk, Russia
- 3 Institute of Cytology and Genetics SB RAS, 630090 Novosibirsk, Russia
The improvement of phenotyping methods is necessary for large-scale screening studies of wheat (Triticum aestivum L.) drought tolerance. The objective of our research was to find out whether it is possible to use chlorophyll (Chl) fluorescence parameters instead of biochemical indicators of drought tolerance when screening wheat. We measured shoot biomass, gas exchange, as well as biochemical and Chl fluorescence indicators in 11 wheat genotypes grown under contrasting water supplies and differing in drought tolerance. The effect of drought on the traits was evaluated using the effect of size index. We made two independent rankings: one based on biochemical indicators and the other on Chl fluorescence parameters. The positions of the three genotypes with the highest comprehensive drought tolerance index in the two independent rankings coincided completely. It is concluded that Chl fluorescence methods are suitable for identifying soft wheat genotypes that differ significantly in their ability to activate cellular defense mechanisms.
Additional key words: ascorbate-glutathione cycle enzymes; chlorophyll fluorescence; comprehensive drought-tolerance index; proline; size of drought effect; sugars.
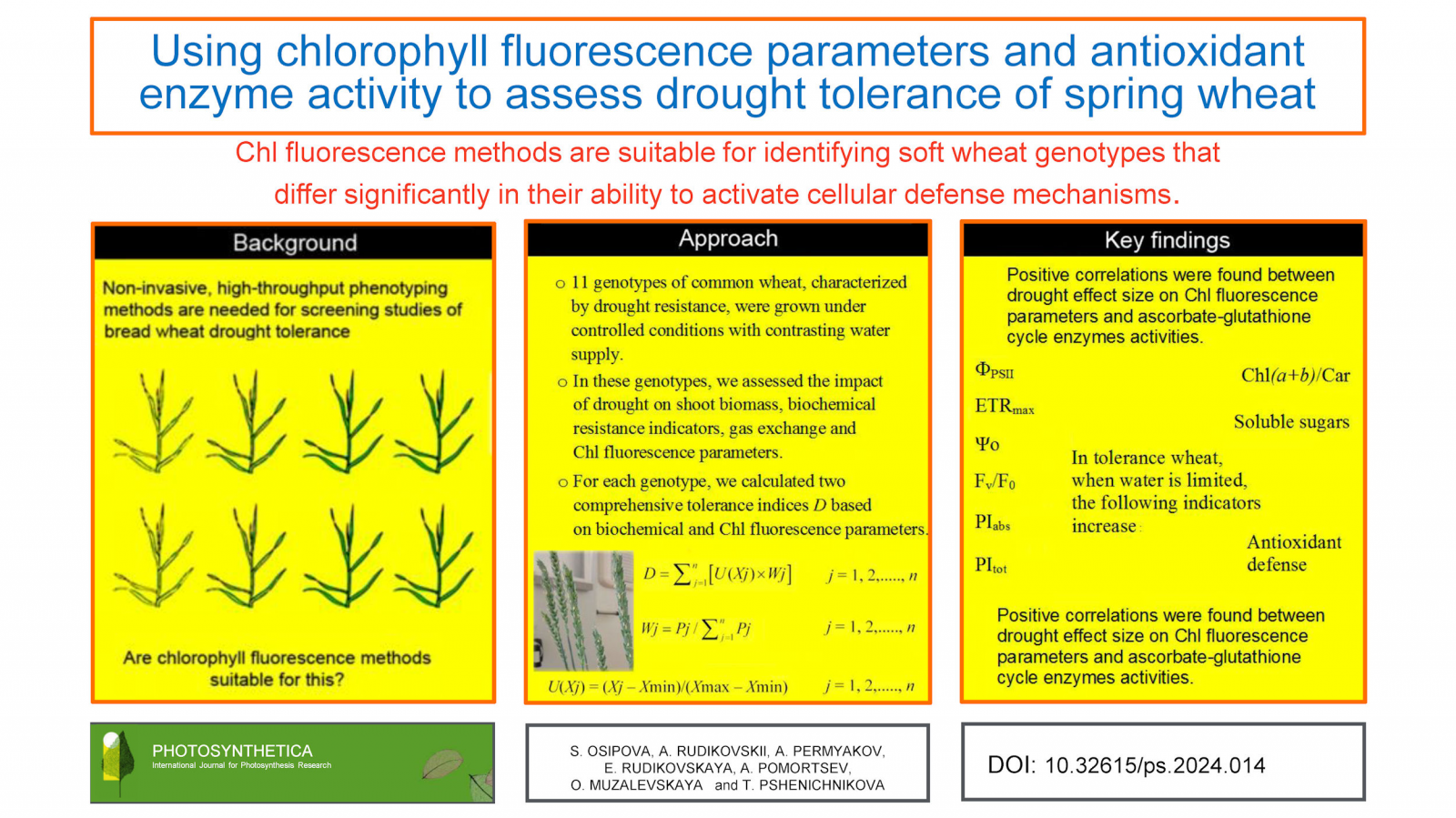
Received: August 31, 2023; Revised: January 22, 2024; Accepted: February 19, 2024; Prepublished online: March 1, 2024; Published: June 27, 2024 Show citation
| ACS | AIP | APA | ASA | Harvard | Chicago | Chicago Notes | IEEE | ISO690 | MLA | NLM | Turabian | Vancouver |
Supplementary files
| Download file | Osipova_3055_supplement-Table_1S.xlsx File size: 46.83 kB |
| Download file | Osipova_3055_supplement-Table_2S.xlsx File size: 10.75 kB |
References
- Baier M., Noctor G., Foyer C., Dietz K.-J.: Antisense suppression of 2-cysteine peroxiredoxin in Arabidopsis specifically enhances the activities and expression of enzymes associated with ascorbate metabolism but not glutathione metabolism. - Plant Physiol. 124: 823-832, 2000.
 Go to original source...
Go to original source... - Bartoli C.G., Guiamet J.J., Kiddle G. et al.: Ascorbate content of wheat leaves is not determined by maximal L-galactono-1,4-lactone dehydrogenase (GalLDH) activity under drought stress. - Plant Cell Environ. 28: 1073-1081, 2005.
 Go to original source...
Go to original source... - Bates L., Waldren R.P., Teare I.D.: Rapid determination of free proline for water-stress studies. - Plant Soil Environ. 39: 205-207, 1973.
 Go to original source...
Go to original source... - Begović L., Galić V., Abićić I. et al.: Implication of intra-seasonal climate variation on chlorophyll a fluorescence and biomass in winter barley breeding program. - Photosynthetica 58: 995-1008, 2020.
 Go to original source...
Go to original source... - Bolouri-Moghaddam M.R., Le Roy K., Xiang L. et al.: Sugar signalling and antioxidant network connections in plant cells. - FEBS J. 277: 2022-2037, 2010.
 Go to original source...
Go to original source... - Botyanszka L., Zivcak M., Chovancek E. et al.: Chlorophyll fluorescence kinetics may be useful to identify early drought and irrigation effects on photosynthetic apparatus in field-grown wheat. - Agronomy 10: 1275, 2020.
 Go to original source...
Go to original source... - Bradford M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. - Anal. Biochem. 72: 248-254, 1976.
 Go to original source...
Go to original source... - Chen K., Chen L., Fan J.B., Fu J.M.: Alleviation of heat damage to photosystem II by nitric oxide in tall fescue. - Photosynth. Res. 116: 21-31, 2013.
 Go to original source...
Go to original source... - D±browski P., Keutgen A.J., Keutgen N. et al.: Photosynthetic efficiency of perennial ryegrass (Lolium perenne L.) seedlings in response to Ni and Cd stress. - Sci. Rep.-UK 13: 5357, 2023.
 Go to original source...
Go to original source... - de Lamotte F., Vianey-Liaud N., Duviau M.-P., Kobrehel K.: Glutathione reductase in wheat grain. 1. Isolation and characterization. - J. Agr. Food Chem. 48: 4978-4983, 2000.
 Go to original source...
Go to original source... - Deng Q., Dou Z., Chen J. et al.: Drought tolerance evaluation of intergeneric hybrids of BC3F1 lines of Saccharum officinarum × Erianthus arundinaceus. - Euphytica 215: 207, 2019.
 Go to original source...
Go to original source... - Dische Z.: Color reactions of carbohydrates. - In: Whistler R.L., Wolfrom M.L. (ed.): Methods in Carbohydrate Chemistry. Pp. 477-512. Academic Press, New York 1962.
- Giannopolitis C.N., Ries S.K.: Superoxide dismutases: 1. Occurrence in higher plants. - Plant Physiol. 59: 309-314, 1977.
 Go to original source...
Go to original source... - Goltsev V.N., Kalaji H.M., Paunov M. et al.: Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. - Russ. J. Plant Physiol. 63: 869-893, 2016.
 Go to original source...
Go to original source... - Hammer Ø., Harper D.A.T., Ryan P.D.: PAST: Paleontological statistics software package for education and data analysis. - Palaeontol. Electron. 4: 4, 2001.
- Hedges L.V., Olkin I.: Estimation of a single effect size: parametric and nonparametric methods. - In: Hedges L.V., Olkin I. (ed.): Statistical Methods for Meta-Analysis. Pp. 75-106. Academic Press, Amsterdam 1985.
 Go to original source...
Go to original source... - Ivanov B.N., Khorobrykh S.A., Kozuleva M.A. et al.: [The role of oxygen and its reactive forms in photosynthesis.] - In: Allahverdiev S.I., Rubin A.B., Shuvalov V.A. (ed.): [Modern Problems of Photosynthesis.] Pp. 407-460. Izhevsk, Moscow 2014. [In Russian]
- Kalaji H.M., Schansker G., Brestic M. et al.: Frequently asked question about chlorophyll fluorescence, the sequel. - Photosynth. Res. 132: 13-66, 2017.
 Go to original source...
Go to original source... - Laxa M., Liebthal M., Telman W. et al.: The role of the plant antioxidant system in drought tolerance. - Antioxidants 8: 94, 2019.
 Go to original source...
Go to original source... - Mwadzingeni L., Shimelis H., Tesfay S., Tsilo T.J.: Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. - Front. Plant Sci. 7: 1276, 2016.
 Go to original source...
Go to original source... - Nakano Y., Asada K.: Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. - Plant Cell Physiol. 22: 867-880, 1981.
 Go to original source...
Go to original source... - Nezhadahmadi A., Prodhan Z.H., Faruq G.: Drought tolerance in wheat. - Sci. World J. 2013: 610721, 2013.
 Go to original source...
Go to original source... - Oguz M.C., Aycan M., Oguz E. et al.: Drought stress tolerance in plants: interplay of molecular, biochemical and physiological responses in important development stages. - Physiologia 2: 180-197, 2022.
 Go to original source...
Go to original source... - Osipova S., Permyakov A., Permyakova M.: Drought tolerance evaluation of bread wheat (Triticum aestivum L.) lines with the substitution of the second homoelogical group chromosomes. - Cereal Res. Commun. 48: 267-273, 2020.
 Go to original source...
Go to original source... - Osipova S.V., Permyakov A.V., Permyakova M.D., Rudikovskaya E.G.: Tolerance of the photosynthetic apparatus in recombinant lines of wheat, adapting to water stress of varying intensity. - Photosynthetica 57: 160-169, 2019.
 Go to original source...
Go to original source... - Panda D., Mishra S.S., Behera P.K.: Drought tolerance in rice: focus on recent mechanisms and approaches. - Rice Sci. 28: 119-132, 2021.
 Go to original source...
Go to original source... - Pandey A.K., Jiang L., Moshelion M. et al.: Functional physiological phenotyping with functional mapping: A general framework to bridge the phenotype-genotype gap in plant physiology. - iScience 24: 102846, 2021.
 Go to original source...
Go to original source... - Perąić V., Ament A., Antunović Dunić J. et al.: PEG-induced physiological drought for screening winter wheat genotypes sensitivity - integrated biochemical and chlorophyll a fluorescence analysis. - Front. Plant Sci. 13: 987702, 2022.
 Go to original source...
Go to original source... - Pleban J.R., Guadagno C.R., Mackay D.S. et al.: Rapid chlorophyll a fluorescence light response curves mechanistically inform photosynthesis modeling. - Plant Physiol. 183: 602-619, 2020.
 Go to original source...
Go to original source... - Pshenichnikova T.A., Osipova S.V., Smirnova O.G. et al.: Regions of chromosome 2A of bread wheat (Triticum aestivum L.) associated with variation in physiological and agronomical traits under contrasting water regimes. - Plants-Basel 10: 1023, 2021.
 Go to original source...
Go to original source... - Rane J., Singh A.K., Tiwari M. et al.: Effective use of water in crop plants in dryland agriculture: implications of reactive oxygen species and antioxidative system. - Front. Plant Sci. 12: 778270, 2022.
 Go to original source...
Go to original source... - Rapacz M., Wójcik-Jagła M., Fiust A. et al.: Genome-wide associations of chlorophyll fluorescence OJIP transient parameters connected with soil drought response in barley. - Front. Plant Sci. 10: 78, 2019.
 Go to original source...
Go to original source... - Sami F., Yusuf M., Faizan M. et al.: Role of sugars under abiotic stress. - Plant Physiol. Biochem. 109: 54-61, 2016.
 Go to original source...
Go to original source... - Sehgal D., Mondal S., Crespo-Herrera L. et al.: Haplotype-based, genome-wide association study reveals stable genomic regions for grain yield in CIMMYT spring bread wheat. - Front. Genet. 11: 589490, 2020.
 Go to original source...
Go to original source... - Sieczko L., D±browski P., Kowalczyk K. et al.: Early detection of phosphorus deficiency stress in cucumber at the cellular level using chlorophyll fluorescence signals. - J. Water Land Dev. SI: 176-186, 2022.
 Go to original source...
Go to original source... - Srivastava A., Biswas S., Yadav S. et al.: Physiological and thylakoid proteome analyses of Anabaena sp. PCC 7120 for monitoring the photosynthetic responses under cadmium stress. - Algal Res. 54: 102225, 2021.
 Go to original source...
Go to original source... - Strasser R.J., Tsimilli-Michael M., Srivastava A.: Analysis of the chlorophyll a fluorescence transient. - In: Papageorgiou G.C., Govindjee (ed.): Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration. Pp. 321-362. Springer, Dordrecht 2004.
 Go to original source...
Go to original source... - Szabados L., Savouré A.: Proline: a multifunctional amino acid. - Trends Plant Sci. 15: 89-97, 2010.
 Go to original source...
Go to original source... - Varshney R.K., Tuberosa R., Tardieu F.: Progress in understanding drought tolerance: from alleles to cropping systems. - J. Exp. Bot. 69: 3175-3179, 2018.
 Go to original source...
Go to original source... - von Wettstein D.: Chlorophyll-letale und der submikroskopische Formwechsel der Plastiden. - Exp. Cell Res. 12: 427-506, 1957. [In German]
 Go to original source...
Go to original source... - Wang J., Zhang X., Han Z. et al.: Analysis of physiological indicators associated with drought tolerance in wheat under drought and re-watering conditions. - Antioxidants 11: 2266, 2022.
 Go to original source...
Go to original source... - Zhang X., Wang Z., Li Y. et al.: Wheat genotypes with higher yield sensitivity to drought overproduced proline and lost minor biomass under severer water stress. - Front Plant Sci. 13: 1035038, 2022.
 Go to original source...
Go to original source... - Zivcak M., Brestic M., Balatova Z. et al.: Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. - Photosynth. Res. 117: 529-546, 2013.
 Go to original source...
Go to original source... - ®ivčák M., Brestič M., Oląovská K., Slamka P.: Performance index as a sensitive indicator of water stress in Triticum aestivum L. - Plant Soil Environ. 54: 133-139, 2008.
 Go to original source...
Go to original source...




