Photosynthetica 2023, 61(4):432-440 | DOI: 10.32615/ps.2023.033
Priming of Pisum sativum seeds with stabilized Pluronic P85 nanomicelles: effects on seedling development and photosynthetic function
- 1 Institute of Biophysics and Biomedical Engineering, Bulgarian Academy of Sciences, Sofia, Bulgaria
- 2 Institute of Plant Physiology and Genetics, Bulgarian Academy of Sciences, Sofia, Bulgaria
- 3 Faculty of Biology, Sofia University 'St. Kliment Ohridsky', Sofia, Bulgaria
- 4 Institute of Plant Biology, Biological Research Centre, Szeged, Hungary
- 5 Institute of Polymers, Bulgarian Academy of Sciences, Sofia, Bulgaria
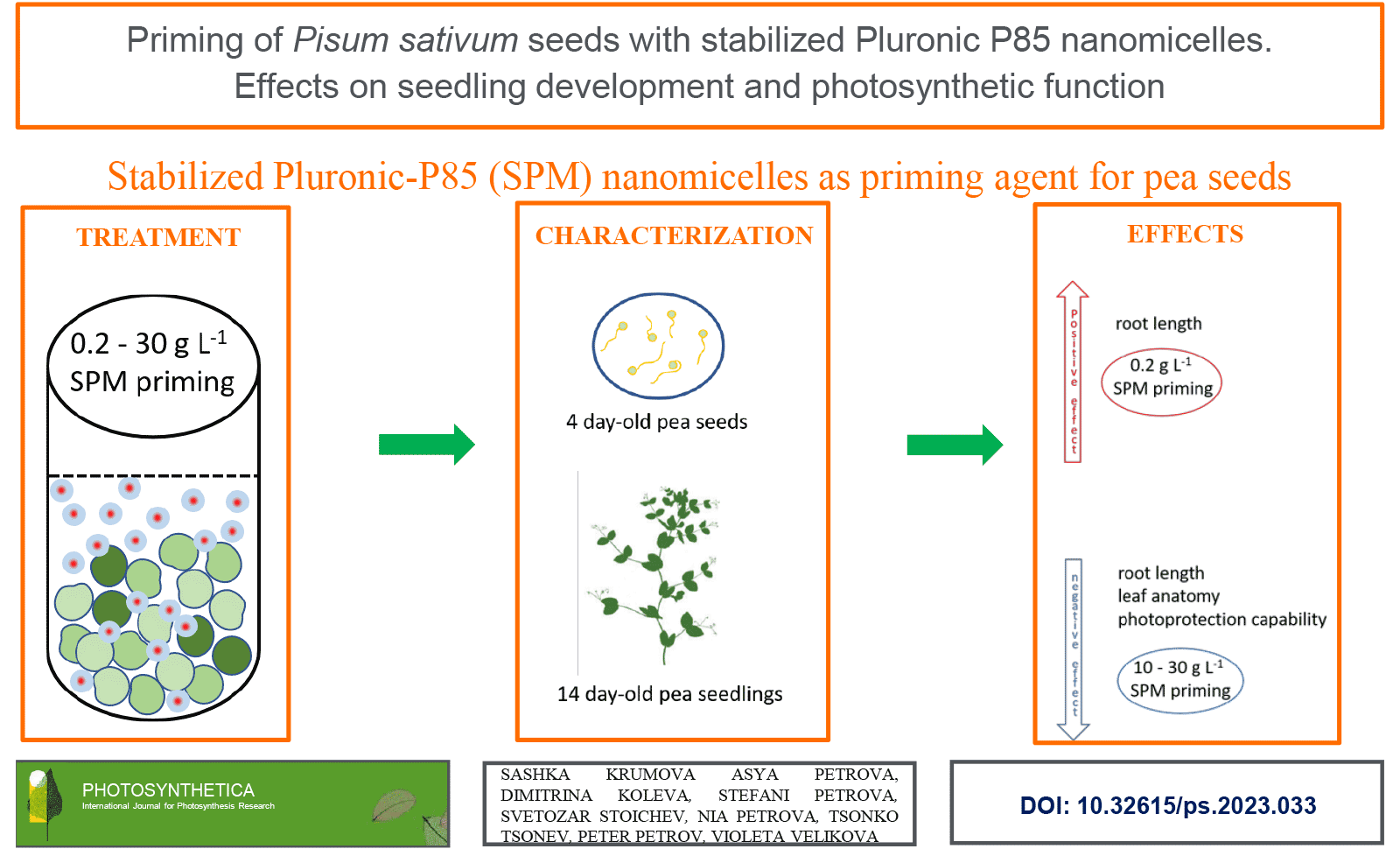
Natural and synthetic polymers are widely explored for improving seed germination and plant resistance to environmental constraints. Here, for the first time, we explore stabilized nanomicelles composed of the biocompatible triblock co-polymer Pluronic P85 (SPM) as a priming agent for Pisum sativum (var. RAN-1) seeds. We tested a wide concentration range of 0.04-30 g(SPM) L-1. Applying several structural and functional methods we revealed that the utilized nanomicelles can positively affect root length, without any negative effects on leaf anatomy and photosynthetic efficiency at 0.2 g L-1, while strong negative effects were recorded for 10 and 30 g(SPM) L-1 concerning root length, leaf histology, and photoprotection capability. Our data strongly suggest that SPM can safely be utilized for seed priming at specific concentrations and are suitable objects for further loading with plant growth regulators.
Additional key words: chlorophyll fluorescence; garden pea; leaf anatomy; nanoparticles; plant biometry; poloxamer.
Received: June 5, 2023; Revised: September 1, 2023; Accepted: September 4, 2023; Prepublished online: September 25, 2023; Published: December 19, 2023 Show citation
| ACS | AIP | APA | ASA | Harvard | Chicago | Chicago Notes | IEEE | ISO690 | MLA | NLM | Turabian | Vancouver |
Supplementary files
| Download file | Krumova_3036_supplement.docx File size: 1.5 MB |
References
- Adhikari K., Mahato G.R., Chen H. et al.: Nanoparticles and their impacts on seed germination. - In: Singh V.P., Singh S., Tripathi D.K. et al. (ed.): Plant Responses to Nanomaterials. Nanotechnology in the Life Sciences. Pp. 21-31. Springer, Cham 2021.
 Go to original source...
Go to original source... - Alakhova D.Y., Kabanov A.V.: Pluronics and MDR reversal: an update. - Mol. Pharm. 11: 2566-2578, 2014.
 Go to original source...
Go to original source... - Almeida M., Magalhães M., Veiga F., Figueiras A.: Poloxamers, poloxamines and polymeric micelles: Definition, structure and therapeutic applications in cancer. - J. Polym. Res. 25: 31, 2018.
 Go to original source...
Go to original source... - An J., Hu P., Li F. et al.: Emerging investigator series: molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. - Environ. Sci.-Nano 7: 2214-2228, 2020.
 Go to original source...
Go to original source... - Anthony P., Davey M.R., Power J.B. et al.: Synergistic enhancement of protoplast growth by oxygenated perfluorocarbon and Pluronic F-68. - Plant Cell Rep. 13: 251-255, 1994.
 Go to original source...
Go to original source... - Anthony P., Jelodar N.B., Lowe K.C. et al.: Pluronic F-68 increases the post-thaw growth of cryopreserved plant cells. - Cryobiology 33: 508-514, 1996.
 Go to original source...
Go to original source... - Anthony P., Lowe K.C., Davey M.R., Power J.B.: Strategies for promoting division of cultured plant protoplast: synergistic effects of haemoglobin (Erythrogen) and Pluronic F-68. - Plant Cell Rep. 17: 13-16, 1997.
 Go to original source...
Go to original source... - Barbulescu D.M., Burton W.A., Salisbury P.A.: Pluronic F-68: an answer for shoot regeneration recalcitrance in microspore-derived Brassica napus embryos. - In Vitro Cell. Dev.-Pl. 47: 282-288, 2011.
 Go to original source...
Go to original source... - Batrakova E.V., Kabanov A.V.: Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. - J. Control. Release 130: 98-106, 2008.
 Go to original source...
Go to original source... - Batrakova E.V., Li S., Vinogradov S.V. et al.: Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. - J. Pharmacol. Exp. Ther. 299: 483-493, 2001.
 Go to original source...
Go to original source... - Borsuk A.M., Roddy A.B., Théroux-Rancourt G., Brodersen C.R.: Structural organization of the spongy mesophyll. - New Phytol. 234: 946-960, 2022.
 Go to original source...
Go to original source... - Brunetti C., Sebastiani F., Tattini M.: Review: ABA, flavonols, and the evolvability of land plants. - Plant Sci. 280: 448-454, 2019.
 Go to original source...
Go to original source... - Cancino G.O., Gill M.I.S., Anthony P. et al.: Pluronic F-68 enhanced shoot regeneration in a potencially novel citrus rootstock. - Artif. Cell. Blood Sub. Biotechnol. 29: 317-324, 2001.
 Go to original source...
Go to original source... - Gago J., Daloso D.M., Carriquí M. et al.: Mesophyll conductance: the leaf corridors for photosynthesis. - Biochem. Soc. T. 48: 429-439, 2020.
 Go to original source...
Go to original source... - Hill M.R., MacKrell E.J., Forsthoefel C.P. et al.: Biodegradable and pH-responsive nanoparticles designed for site-specific delivery in agriculture. - Biomacromolecules 16: 1276-1282, 2015.
 Go to original source...
Go to original source... - Iordan-Costache M., Lowe K.C., Davey M.R., Power J.B.: Improved micropropagation of Populus spp. by Pluronic F-68. - Plant. Growth Regul. 17: 233-239, 1995.
 Go to original source...
Go to original source... - Janská A., Pecková E., Sczepaniak B. et al.: The role of the testa during the establishment of physical dormancy in the pea seed. - Ann. Bot.-London 123: 815-829, 2019.
 Go to original source...
Go to original source... - Jarak I., Varela C.L., da Silva E.T. et al.: Pluronic-based nanovehicles: Recent advances in anticancer therapeutic applications. - Eur. J. Med. Chem. 206: 112526, 2020.
 Go to original source...
Go to original source... - Jeong B.: Injectable biodegradable materials. - In: Vernon B. (ed.): Injectable Biomaterials. Pp. 323-337. Woodhead Publishing, Cambridge 2011.
 Go to original source...
Go to original source... - Johnsson M., Silvander M., Karlsson G., Edwards K.: Effect of PEO-PPO-PEO triblock copolymers on structure and stability of phosphatidylcholine liposomes. - Langmuir 15: 6314-6325, 1999.
 Go to original source...
Go to original source... - Kaparakis G., Alderson P.G.: Enhancement of in vitro cell proliferation of pepper (Capsicum annuum L.) by Pluronic F-68, haemoglobin and arabinogalactan proteins. - J. Hortic. Sci. Biotech. 78: 647-649, 2003.
 Go to original source...
Go to original source... - Khatun A., Naher Z., Mahboob S. et al.: An efficient protocol for plant regeneration from the cotyledons of kenaf (Hibiscus cannabinus L.). - Biotechnology 2: 86-93, 2003.
 Go to original source...
Go to original source... - Khehra M., Lowe K.C., Davey M.R., Power J.B.: An improved micropropagation system for Chrysanthemum based on Pluronic F-68-supplemented media. - Plant Cell Tiss. Org. Cult. 41: 87-90, 1995.
 Go to original source...
Go to original source... - Kok A.D.-X., Mohd Yusoff N.F., Sekeli R. et al.: Pluronic F-68 improves callus proliferation of recalcitrant rice cultivar via enhanced carbon and nitrogen metabolism and nutrients uptake. - Front. Plant Sci. 12: 667434, 2021.
 Go to original source...
Go to original source... - Krumova S., Petrova A., Petrova N. et al.: Seed priming with single-walled carbon nanotubes grafted with Pluronic P85 preserves the functional and structural characteristics of pea plants. - Nanomaterials 13: 1332, 2023.
 Go to original source...
Go to original source... - Kumar V., Laouar M.R., Davey B.J. et al.: Pluronic F-68 stimulates growth of Solanum dulcamara in culture. - J. Exp. Bot. 43: 487-493, 1992.
 Go to original source...
Go to original source... - Kurczyñska E., Godel-Jêdrychowska K., Sala K., Milewska-Hendel A.: Nanoparticles-plant interaction: What we know, where we are? - Appl. Sci. 11: 5473, 2021.
 Go to original source...
Go to original source... - Kwiatkowski T.A., Rose A.L., Jung R. et al.: Multiple poloxamers increase plasma membrane repair capacity in muscle and nonmuscle cells. - Am. J. Physiol.-Cell Physiol. 318: C253-C262, 2020.
 Go to original source...
Go to original source... - Lee S.-Y., Kim D.-I.: Stimulation of murine granulocite macrophage-colony stimulation factor production by Pluronic F-68 and polyethylene glycol in transgenic Nicotiana tabacum cell culture. - Biotechnol. Lett. 24: 1779-1783, 2002.
 Go to original source...
Go to original source... - Lehmeier C., Pajor R., Lundgren M.R. et al.: Cell density and airspace patterning in the leaf can be manipulated to increase leaf photosynthetic capacity. - Plant J. 92: 981-994, 2017.
 Go to original source...
Go to original source... - Lowe K.C., Anthony P., Davey M.R. et al.: Enhanced protoplast growth at the interface between oxygenated fluorocarbon liquid and aqueous culture medium supplemented with Pluronic F-68. - Artif. Cell. Blood Sub. Biotechnol. 23: 417-422, 1995.
 Go to original source...
Go to original source... - Metcalfe C.R., Chalk L.: Anatomy of Dicotyledons. Vol. I: Systematic Anatomy of Leaf and Stem, with a Brief History of the Subject. Pp. 288. Clarendon Press, Oxford 1979.
- Nalewaja J.D., Praczyk T., Matysiak R.: Nitrogen fertilizer, oil, and surfactant adjuvants with nicosulfuron. - Weed Technol. 12: 585-589, 1998.
 Go to original source...
Go to original source... - Nugraha D.H., Anggadiredja K., Rachmawati H.: Mini-review of poloxamer as a biocompatible polymer for advanced drug delivery. - Braz. J. Pharm. Sci. 58: e21125, 2022.
 Go to original source...
Go to original source... - Ottenbrite R.M., Javan R.: Biological structures. - In: Bassani F., Liedl G.L., Wyder P. (ed.): Encyclopedia of Condensed Matter Physics. Pp. 99-108. Elsevier, Oxford 2005.
 Go to original source...
Go to original source... - Pereira A.E.S., Oliveira H.C., Fraceto L.F.: Polymeric nanoparticles as an alternative for application of gibberellic acid in sustainable agriculture: a field study. - Sci. Rep.-UK 9: 7135, 2019.
 Go to original source...
Go to original source... - Petrov P., Bozukov M., Tsvetanov C.B.: Innovative approach for stabilizing poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) micelles by forming nano-sized networks in the micelle. - J. Mater. Chem. 15: 1481-1486, 2005.
 Go to original source...
Go to original source... - Ranal M.A., de Santana D.G., Ferreira W.R., Mendes-Rodrigues C.: Calculating germination measurements and organizing spreadsheets. - Braz. J. Bot. 32: 849-855, 2009.
 Go to original source...
Go to original source... - Schärtl W.: Light Scattering from Polymer Solutions and Nanoparticle Dispersions. Pp. 191. Springer, Berlin-Heidelberg 2007.
- Smith W.K., Vogelmann T.C., DeLucia E.H. et al.: Leaf form and photosynthesis: do leaf structure and orientation interact to regulate internal light and carbon dioxide? - BioScience 47: 785-793, 1997.
 Go to original source...
Go to original source... - Szġllġsi R., Molnár Á., Kondak S., Kolbert Z.: Dual effect of nanomaterials on germination and seedling growth: stimulation vs. phytotoxicity. - Plants-Basel 9: 1745, 2020.
- Terashima I., Hanba Y.T., Tholen D. et al.: Leaf functional anatomy in relation to photosynthesis. - Plant Physiol. 155: 108-116, 2011.
 Go to original source...
Go to original source... - Théroux-Rancourt G., Roddy A.B., Earles J.M. et al.: Maximum CO2 diffusion inside leaves is limited by the scaling of cell size and genome size. - Proc. R. Soc. B 288: e20203145, 2021.
 Go to original source...
Go to original source... - Velikova V., Arena C., Izzo L.G. et al.: Functional and structural leaf plasticity determine photosynthetic performances during drought stress and recovery in two Platanus orientalis populations from contrasting habitats. - Int. J. Mol. Sci. 21: 3912, 2020.
 Go to original source...
Go to original source... - Velikova V., Petrova N., Kovács L. et al.: Single-walled carbon nanotubes modify leaf micromorphology, chloroplast ultrastructure and photosynthetic activity of pea plants. - Int. J. Mol. Sci. 22: 4878, 2021.
 Go to original source...
Go to original source... - Vinzant K., Rashid M., Khodakovskaya M.V.: Advanced applications of sustainable and biological nano-polymers in agricultural production. - Front. Plant Sci. 13: 1081165, 2023.
 Go to original source...
Go to original source... - Wang J., Segatori L., Biswal S.L.: Probing the association of triblock copolymers with supported lipid membranes using microcantilevers. - Soft Matter 10: 6417-6424, 2014.
 Go to original source...
Go to original source... - Xin X., He Z., Hill M.R. et al.: Efficiency of biodegradable and pH-responsive polysuccinimide nanoparticles (PSI-NPs) as smart nanodelivery systems in grapefruit: in vitro cellular investigation. - Macromol. Biosci. 18: e1800159, 2018.
 Go to original source...
Go to original source... - Xin X., Judy J.D., Sumerlin B.B., He Z.: Nano-enabled agriculture: from nanoparticles to smart nanodelivery systems. - Environ. Chem. 17: 413-425, 2020a.
 Go to original source...
Go to original source... - Xin X., Zhao F., Rho J.Y. et al.: Use of polymeric nanoparticles to improve seed germination and plant growth under copper stress. - Sci. Total Environ. 745: 141055, 2020c.
 Go to original source...
Go to original source... - Xin X., Zhao F., Zhao H. et al.: Comparative assessment of polymeric and other nanoparticles impacts on soil microbial and biochemical properties. - Geoderma 367: 114278, 2020b.
 Go to original source...
Go to original source... - Yu J., Qiu H., Yin S. et al.: Polymeric drug delivery system based on pluronics for cancer treatment. - Molecules 26: 3610, 2021.
 Go to original source...
Go to original source... - Zhang W., Coughlin M.L., Metzger J.M. et al.: Influence of cholesterol and bilayer curvature on the interaction of PPO-PEO block copolymers with liposomes. - Langmuir 35: 7231-7241, 2019.
 Go to original source...
Go to original source... - Zhirnov A.E., Demina T.V., Krylova O.O. et al.: Lipid composition determines interaction of liposome membranes with Pluronic L61. - BBA-Biomembranes 1720: 73-83, 2005.
 Go to original source...
Go to original source...




